CPT-DIR
Continues-sPatial-Temporal DIR (CPT-DIR) is an advanced toolbox for deformable image registration in radiotherapy. Used for intra-fractional motion estimation and inter-fractional anatomic changes.
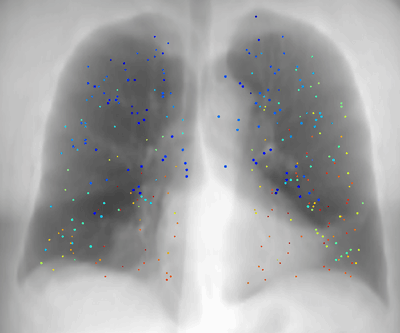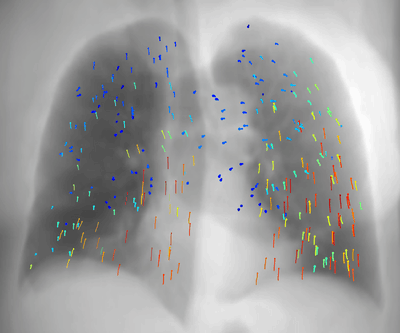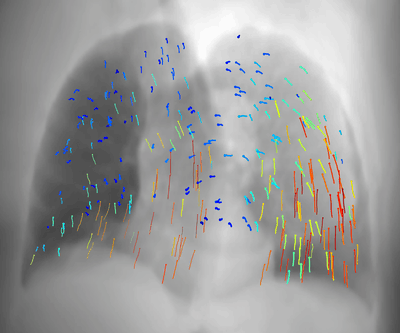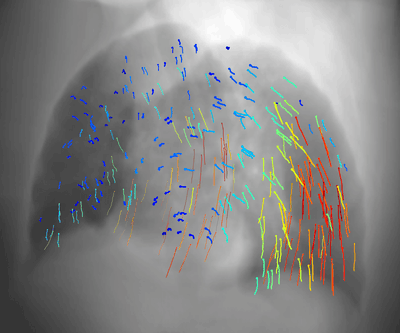
Latest News
Publications
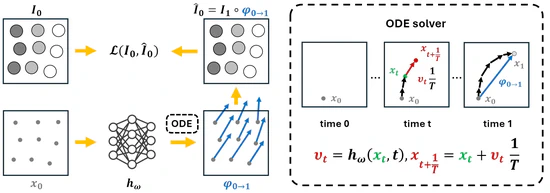
CPT-Interp: Continuous sPatial and Temporal Motion Modeling for 4D Medical Image Interpolation
In this work, we introduce a novel Continuous sPatial and Temporal (CPT) modeling approach for 4D medical image interpolation, addressing the limitations of discrete motion modeling in both spatial and temporal domains. By leveraging implicit neural representations and integrating an ODE solver, our method achieves superior accuracy and speed without requiring extensive training datasets or domain-specific fine-tuning. Through comprehensive experiments and ablation studies, we demonstrated the contributions of spatial and temporal continuity, as well as the robustness of our method across different datasets. CPT-Interp not only outperforms state-of-the-art methods but also exhibits remarkable generalization ability, making it a promising solution for clinical applications.
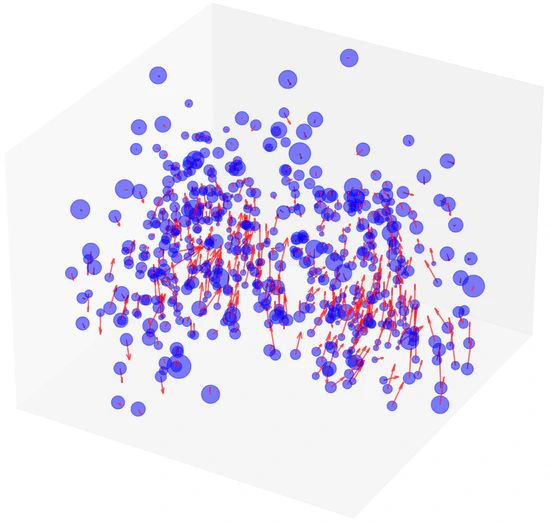
Gaussian Representation for Deformable Image Registration
This paper presented a novel deformable image registration (DIR) technique using parametric 3D Gaussian control points to model continuous spatial deformation fields effectively. By integrating transformation vectors with linear blend skinning (LBS) for voxel motion interpolation, our approach simplifies computational demands while ensuring high accuracy and efficiency. The adaptive density of Gaussian points further allows for precise handling of varying motion complexities.
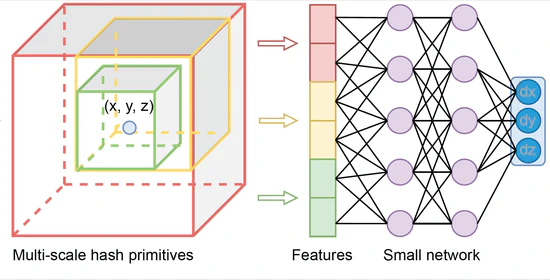
Neural Graphics Primitives-based Deformable Image Registration for On-the-fly Motion Extraction
In this study, we have successfully integrated NGP into DIR, a novel contribution that significantly enhances the accuracy and efficiency of medical image alignment as demonstrated on the DIR-lab dataset. The NGPDIR framework exhibits robust performance across various metrics, particularly in landmark alignment precision and the accommodation of anatomical sliding boundaries. This advancement not only propels the DIR field forward but also opens new avenues for real-time clinical applications, potentially transforming patient care with its rapid, reliable imaging capabilities.
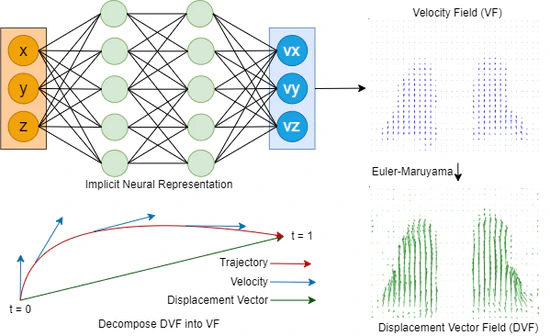
Beyond Voxel-Based Methods: Continuous Motion Modeling for Enhanced Deformable Image Registration
Our novel approach, capitalizing on the power of INR, offers a means to model motion in a continuous manner for intra-fraction motion modelling. By representing the registration process as a continuous flow, we circumvent the limitations inherent to the classic voxel-based representations, particularly the dilemma of discretization. The tangible enhancements are evident: both INR-DIR (E2E and LDD) methods consistently outperformed classic B-splines regarding TRE, MAE, landmark, and Dice coefficients. Remarkably, the registration times were slashed by more than half. One of the standout features of our LDD method is its ability to handle reverse trajectories, a capability unattainable with prior techniques. This promises more robust and versatile image registration applications in radiotherapy.
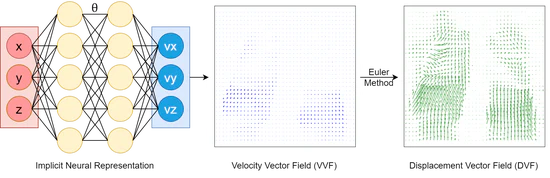
Continuous sPatial-Temporal Deformable Image Registration (CPT-DIR) for motion modelling in radiotherapy: beyond classic voxel-based methods
In summary, the innovative CPT-DIR approach, integrating principles of INR and LDDMM, represents a11 significant departure from traditional voxel-based methods in DIR. By adopting a paradigm of continuous motion modelling, we transcend the limitations inherent in voxel-based representations, offering a more robust, automatic and versatile solution. Leveraging spatial continuity, we effectively handle the intricacies of sliding organ boundaries, while temporal continuity alleviates the complexities associated with significant anatomical changes over time. The tangible benefits are evident in its superior performance compared to classic B-splines methods. CPT-DIR consistently achieves better performance by all kinds of evaluation matrices. Additionally, the efficiency gains are substantial, with registration times slashed by more than half.
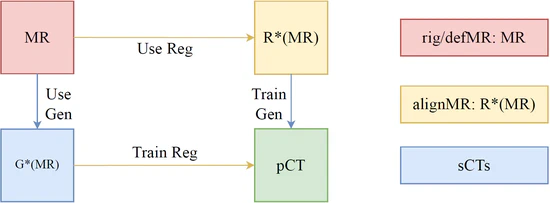
A Unified Generation-Registration Framework for Improved MR-based CT Synthesis in Proton Therapy
In this study, we developed an approach that substantially improves the anatomical accuracy of synthetic CT (sCT) images for MR-based radiotherapy planning. By integrating image generation with the deformable registration processes, the novel framework effectively addresses the inherent challenges in MR-CT registration and sCT evaluation. The methodological synergy of generation and registration networks, coupled with a strategic approach to proton dose planning, contributes to enhancing treatment planning accuracy. This study represents a meaningful step forward in the application of MR-based proton treatment planning and daily adaptive replanning, offering potential improvements in reducing the imaging-related dose and patient outcomes in the field of radiotherapy.


